DNA methylation tests enable better colorectal cancer treatment - BGI Perspectives
2022-12-05
With the purpose of deepening knowledge on colorectal cancer, we invited Yantao Li and Zhe Wang, respectively Director of Cancer Early Screening Products and Product Director of Oncology at BGI Genomics. During our conversation, they also highlight the importance of incidences and preventive screening.
What is colorectal cancer (CRC)?
Yantao Li: Colorectal cancer is a disease in which cells in the colon or rectum grow out of control. Sometimes it is called colon cancer for short. The colon is the large intestine or large bowel. The rectum is the passageway that connects the colon to the anus. Sometimes abnormal growths, called polyps, form in the colon or rectum. Over time, some polyps may turn into cancer.

Yantao Li, BGI Genomics Director of Cancer Early Screening Products
Can you explain the global impact of this form of cancer?
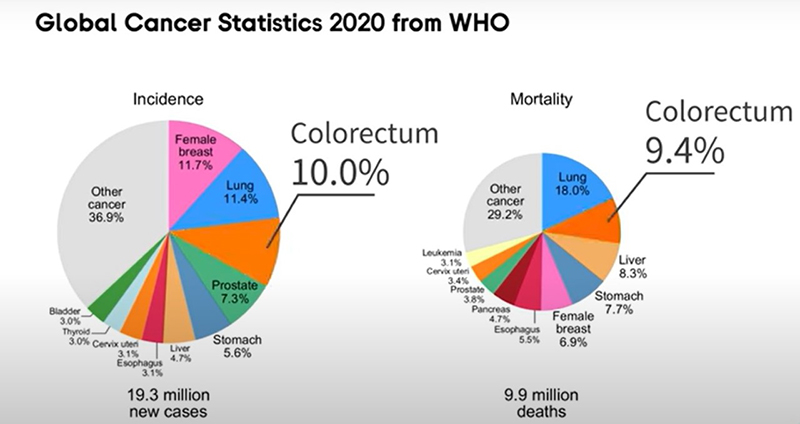
Yantao Li: As the third most common malignancy and the second most deadly cancer, colorectal cancer (CRC) induced an estimated 1.9 million incidence cases and 0.9 million deaths worldwide in 2020. In 2020, CRC accounted for 10% of global cancer incidence and 9.4% of cancer deaths, just lower than lung cancer, which comprises 18% of deaths. The global number of new CRC cases is predicted to reach 3.2 million in 2040, based on the projection of aging, population growth, and human development.
Why are the incidence rates of CRC increasing?
Yantao Li: The increase in CRC incidence is mainly attributed to the elevated exposure to environmental risk factors resulting from shifting lifestyle and diet toward westernization (for example, obesity, physical inactivity, poor diets, alcohol drinking and smoking). As such, the burden of CRC is shifting towards low-income and middle-income countries as they become westernized. Moreover, CRC incidence rates are uniquely increasing in young adults in high-income countries (Germany, USA, Australia, Canada, New Zealand, UK, Denmark, Slovenia and Sweden) across North America, Europe, and Oceania, where rates in older adults are stable or declining.
What areas of the globe are most heavily affected by CRC?
Yantao Li: At the country level, Hungary, Slovakia, Norway, the Netherlands, and Denmark, have the highest age-standardized incidence rates, with rates of 45.3, 43.9, 41.9, 41.0, and 40.9 cases per 100,000 persons, respectively, in 2020. China and the United States have the highest estimated number of new CRC cases in 2020. In addition, the Russian Federation, India, Germany, Brazil, the United Kingdom, Italy, and France are also among the top 10 countries with the highest incidence cases of CRC in 2020.
Why is CRC screening so important?
Yantao Li: Early stages of colorectal cancer usually present no symptoms. Symptoms tend to appear as cancer progresses. Many colorectal cancers can be prevented through regular screening. Screening can find precancerous polyps — abnormal growths in the colon or rectum — so that they can be removed before they turn into cancer.
Screening is important because, when found early, colorectal cancer is highly treatable. If colorectal cancer is detected early, the five-year relative survival rate of patients can be as high as 90%. Conversely, if it is stage IV at the time of discovery, the five-year survival rate will only be 10%.
Who should be screening for CRC?
Yantao Li: When it comes to starting CRC screening, guidelines for individuals can differ between countries. According to the American College of Gastroenterology CRC screening guidelines, CRC screening in average-risk individuals should start at the age of 45. Most people should begin screening for colorectal cancer soon after turning 45, and continue getting screened at regular intervals.
However, you may need to be tested earlier than 45, or more often than other people, if you have inflammatory bowel diseases such as Crohn’s disease or ulcerative colitis, a personal or family history of colorectal cancer or colorectal polyps, or a genetic syndrome such as familial adenomatous polyposis (FAP)external icon or hereditary non-polyposis colorectal cancer (Lynch syndrome).
Can you explain some of the current CRC screening methods?
Yantao Li: Several screening tests can be used to find polyps or colorectal cancer, such as stool tests, flexible sigmoidoscopy, CT colonography and colonoscopy. If the test result is positive or abnormal on some screening tests (stool tests, flexible sigmoidoscopy, and CT colonography), a diagnosed colonoscopy test is needed to complete the screening process.
1) Stool-based Tests: The guaiac-based fecal occult blood test (gFOBT) uses the chemical guaiac to detect blood in the stool. It is done once a year. The fecal immunochemical test (FIT) uses antibodies to detect blood in the stool. It is also done once a year in the same way as a gFOBT. The FIT-DNA test (also referred to as the stool DNA test) combines the FIT with a test that detects altered DNA in the stool. For this test, you collect an entire bowel movement and send it to a lab, where it is checked for altered DNA and for the presence of blood. It is done once every three years.
2) Flexible Sigmoidoscopy: For this test, the doctor puts a short, thin, flexible, lighted tube into your rectum and checks for polyps or cancer inside the rectum and lower third of the colon. It is done every 5 or 10 years with a FIT every year.
3) Colonoscopy: This is similar to flexible sigmoidoscopy, except the doctor uses a long, thin, flexible, lighted tube to check for polyps or cancer inside the rectum and the entire colon. During the test, the doctor can find and remove most polyps and some cancers. Colonoscopy also is used as a follow-up test if anything unusual is found during one of the other screening tests. It is done every ten years (for people who do not have an increased risk of colorectal cancer).

Zhe Wang, BGI Genomics Product Director of Oncology
What is the DNA methylation test as it relates to CRC screening?
Zhe Wang: DNA methylation refers to the covalent bonding of a methyl group at the 5C position of the cytosine of the CpG dinucleotide in the genome under the action of DNA methyltransferase. Colorectal cancer (CRC) arises from the accumulation of genetic and epigenetic alterations. DNA methylation is one of the most important epigenetic events to occur during the early stages of such oncogenic transformation. The methylation status of some genes is significantly different in colorectal cancer patients and healthy individuals.

COLOTECT™ developed by BGI Genomics
What is unique about the DNA methylation test offered by COLOTECT™?
Zhe Wang: DNA methylation biomarkers of COLOTECT™ are developed based on whole-genome bisulfite sequencing results of colorectal cancer.
Who can conduct one of these tests, and how easy is it to use?
Zhe Wang: COLOTECT™ applies to all people aged 40~75 who need colorectal cancer screening. COLOTECT™ can be completed in the comfort of your home and does not require special preparation, dietary or medication changes, sedation, or time off from work. Then the samples can be mailed directly to the laboratories.
Can you explain the sample analysis procedure and how does this lead to an accurate test result?
Zhe Wang: The COLOTECT™ 3.0 sample collection kit contains two sample tubes, one for each of the two different tests.
One test is a DNA extraction from one of your stool samples and DNA methylation biomarkers detection for colorectal cancer and/or advanced precancerous lesions. The other test is a fecal immunochemical (FIT) test, which detects fecal hemoglobin from the other one of your stool samples.
The combined DNA methylation and protein biomarkers mean that COLOTECT™ 3.0 has better sensitivity than FIT for CRC and advanced precancerous lesions. COLOTECT™ 3.0 has a 96.08% sensitivity for CRC and an 88.7% specificity for gauging non-advanced adenomas. When it comes to detecting patients with advanced precancerous lesions, the sensitivity is 52.5%.
How difficult is it to encourage the general public not to be embarrassed by CRC testing?
Zhe Wang: CRC screening programs have been implemented in various countries. However, the participation rate remains disappointingly low. Some of the barriers to CRC testing include:
- Fear and denial around the test outcome
- A misconception that the test is not applicable if you do not have any apparent symptoms of bowel cancer
- Concerns about the practicalities and cleanliness of the test
- Individual perceived risk being low or consideration of future consequences of colorectal cancer
- The fact that it takes place away from the usual healthcare settings
- Low health literacy and numeracy
- Gender - on the whole, males were less likely to take part in screening
- People from the lower socioeconomic group.
Some people face barriers after deciding to participate in colorectal cancer screening and receiving a positive FIT and may decide not to attend further tests (usually colonoscopy).
Health professionals may have a role in providing information to support people in making an informed decision about attending any further tests and overcome formerly mentioned and other barriers such as:
- Concerns about the procedure (concerns about doing the bowel preparation and fear about pain and discomfort)
- Anxiety and denial
- Cognitive abilities and ability to make an informed decision
- Perceived risk and perceived mortality
Zhe Wang: COLOTECT™ is a non-invasive test. Patients can collect stool samples at home. COLOTECT™ is especially useful when it comes to:
- Avoiding the loss of time away from work or family duties
- Allowing for patients without access to endoscopy services to undergo CRC screening (Underserved or rural communities)
- Allowing patients that are nervous about engaging with a healthcare facility to undergo CRC screening (COVID pandemic)
Screening and detection of CRC and precancerous lesions are important to improve CRC patients' diagnosis and survival rates. COLOTECT™ 3.0 had a 96.08 percent sensitivity for CRC. In detecting patients with advanced precancerous lesions, sensitivity was 52.5 percent for COLOTECT™ 3.0, which was found to be superior to FIT, with a sensitivity of 22.5 percent.
Can COLOTECT™ help combat rising CRC rates in developing countries?
Zhe Wang: In developing countries, healthcare expenditure is relatively low. COLOTECT™ can be used as an accurate and cost-effective stool test for primary screening, which can help to improve the compliance rate, find those high-risk patients for further colonoscopy diagnosis precisely, and maximize the use of limited healthcare resources.
Moreover, COLOTECT™ has tested over 400,000 people in China, and BGI Genomics is very experienced in the organization, mobilization and education of CRC screening programs, which can help to implement the CRC program in developing countries.
Where can readers find more information?
Please refer to the official website of COLOTECT™ : https://colotectglobal.com/
Additional Reading:
BGI Genomics 2023 Global State of Colorectal Cancer Awareness Report
About Yantao Li, PhD
Yantao Li is the Director of Cancer Early Screening Products and has been engaged in health-related research and work for more than eight years. He also likes fitness training and outdoor sports and is a CrossFit Level 1 certified trainer.
About Zhe Wang
Zhe Wang is the Product Director of the Oncology Department at BGI Genomics and has been dedicated to working in translational medicine by using cutting-edge genomics research results to develop more efficient genetic tests to help doctors to improve their clinical practice. For example, the development of carrier screening tests for monogenetic diseases, chromosomal abnormalities tests of miscarriage, preimplantation genetic tests of embryo, genetic tests of precision oncology and cancer screening tests.
About BGI Genomics:
BGI Genomics, headquartered in Shenzhen China, is the world’s leading integrated solutions provider of precision medicine. Our services cover more than 100 countries and regions, involving more than 2,300 medical institutions. In July of 2017, as a subsidiary of BGI Group, BGI Genomics (300676.SZ) was officially listed on the Shenzhen Stock Exchange. BGI has topped the Asia Pacific and China life science corporate institution ranking table for the seventh year running, released in the 2022 Nature Index Annual Tables.