New Combined Therapy Shows Promise for Advanced Stomach Cancer Treatment | Nature Communications
2024-10-15
 source: Nature Communications
source: Nature Communications
Recent clinical research suggested that Nivolumab and Anlotinib as new combination therapy have shown promising treatment results in advanced stomach and esophageal cancer. The research was conducted by Zhongshan Hospital, Fudan University, and BGI Genomics and published in Nature Communications Journal in October 2024.
Upper gastrointestinal tract cancers, such as gastric adenocarcinoma (GAC) and esophageal squamous cell carcinoma (ESCC), rank among the top seven most common cancers worldwide and are often diagnosed at a late stage. Advanced forms are difficult to treat, especially when initial therapies fail or cause intolerable side effects, necessitating second-line treatments.
The trial investigated combining two drugs—Nivolumab and Anlotinib—to treat patients with advanced GAC and ESCC. Nivolumab is an immunotherapy that helps the immune system target cancer cells, while Anlotinib blocks signals that enable tumors to grow new blood vessels. Researchers believed that combining these drugs would boost the body's ability to fight cancer while slowing tumor growth.
Positive Response with Manageable Side Effects
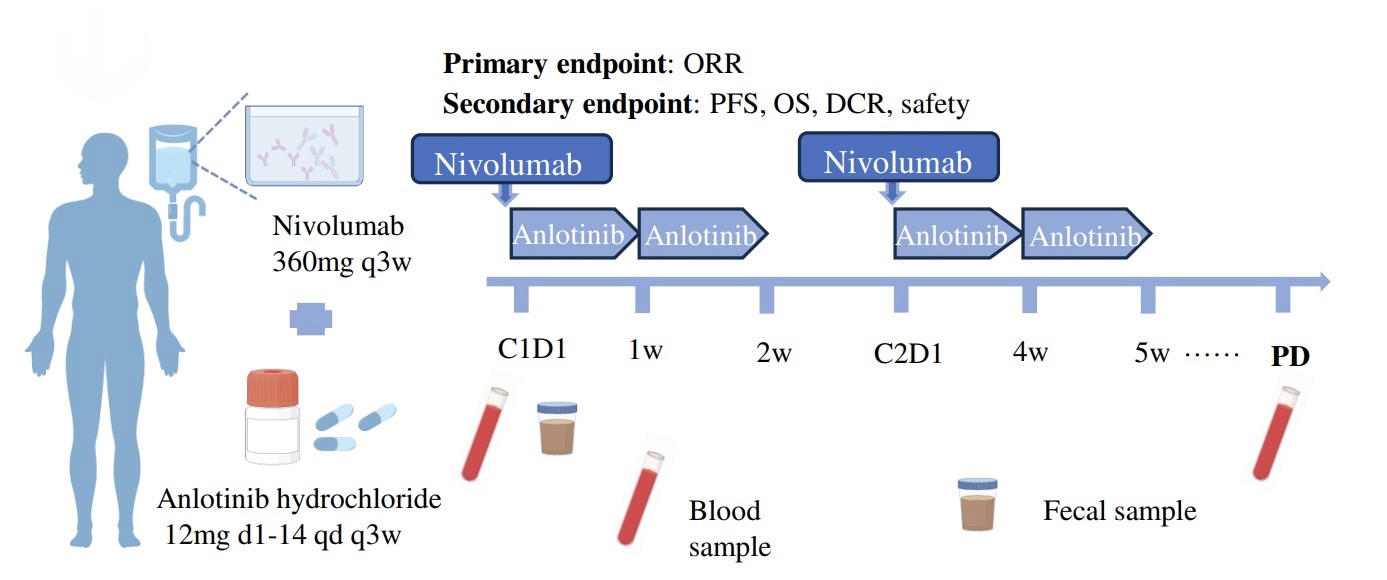
Figure 1. Schematic overview of the therapeutic regimen.
The trial enrolled 48 patients, including 45 with GAC and 3 with ESCC. The overall response rate (ORR) was 29.2%, indicating that nearly one-third of patients saw a significant reduction in tumor size. Additionally, the disease control rate (DCR) reached 64.6%, meaning most patients experienced some degree of stability in their condition.
The median progression-free survival (PFS), which measures the time patients live without disease progression, was 4.0 months. The median overall survival (OS), representing the average time patients lived after starting the treatment, was 11.1 months. This outcome compares favorably with historical data from other late-line therapies in similar cancers.
The combination therapy showed a manageable safety profile, with common treatment-related side effects being hypertension, hypothyroidism, and mild liver dysfunction. While some patients experienced more severe side effects, such as intestinal obstruction, the incidence of grade 3 or 4 side effects was relatively low, making this therapy an attractive option for patients with limited alternatives.
Biomarkers for Potential Clinical Reference
The study found that genomic analysis of the majority of gastric adenocarcinoma patients has identified high-frequency mutations in genes such as TP53, NCOR2, LRP1B, and MUC16. These mutations exhibited a significant degree of concordance between tissue samples and circulating tumor DNA (ctDNA), indicating that ctDNA can effectively reflect the genetic characteristics of tumors.
Notably, the study established a substantial correlation between pretreatment variant allele frequency (VAF) in ctDNA and overall patient survival. Additionally, research has demonstrated that VAF levels in ctDNA tend to increase as disease progression occurs. This suggests that continuous monitoring of these variants may enable clinicians to more accurately predict patient responses to treatment and tailor therapeutic regimens to better address disease status changes.
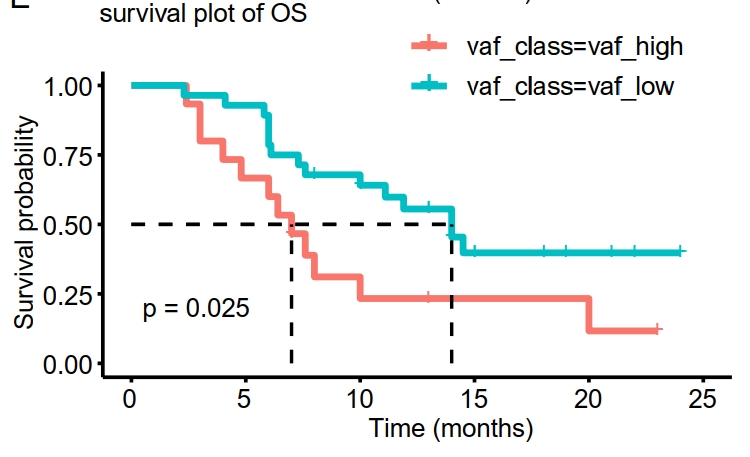
Figure 2. Kaplan–Meier analysis of OS in patients stratified by ≥5% versus <5% pretreatment mean-VAF.
Researchers further explored how proteins in the blood could help predict which gastric adenocarcinoma (GAC) patients respond best to the treatment combination. Proteomic profiling result shows that patients who responded well to treatment had lower levels of proteins like IL-6, IL-8, and MUC16 compared to non-responders. Higher levels of these proteins, especially IL-6, CSF-1, and IL-8, were linked to worse survival rates. While promising, these results highlight the need for more research to fully understand the complex immune responses at play.
Researchers also made a promising discovery: the association of CD68+ PD-L1+ PD-1+ macrophages with improved survival, positing these macrophages as potential harbingers of a positive therapeutic response.
The exploratory analyses revealed that the presence of a pre-existing immune signature characterized by a high percentage of CD68+PD-L1+PD-1+ macrophages and low pretreatment VAF, as well as low expression of certain cytokines were significantly associated with improved clinical outcomes in patients with GAC.
Dr. An Guo, Clinical Research Manager from BGI Genomics, co-author of the study, shared his thoughts: “This study elucidates that the combination of immunotherapy and anti-vascular therapy can serve as a viable treatment strategy for advanced gastric and esophageal cancer beyond second-line therapy, thereby holding significant implications for large-scale clinical investigations. “
He further emphasized the next steps, “Moving forward, the research team intends to further validate the efficacy of this combinatorial approach in an extensive multi-center clinical trial while also continuing to explore the role of biomarkers in predicting therapeutic outcomes, ultimately aiming to provide patients with more precise and personalized treatment options.”
The new combination therapy has shown promise in treating advanced gastric and esophageal cancers. With a manageable safety profile, improved survival outcomes, and insights into personalized medicine, this study represents a step forward in the fight against these challenging cancers. It offers hope for patients and families looking for better options when standard treatments are no longer effective.
BGI SENTIS™ Cancer+ Discovery Panel
By utilizing ctDNA from the peripheral blood of patients with advanced tumors as samples, and employing next-generation sequencing technology, the BGI SENTIS™ Cancer+ Discovery Panel offers a variety of options. These include targeted panels that analyze genes directly linked to specific cancers based on well-documented scientific research, as well as larger cancer panels designed for broader cancer analysis and discovery.
About BGI Genomics
BGI Genomics, headquartered in Shenzhen, China, is the world's leading integrated solutions provider of precision medicine. Its services cover more than 100 countries and regions, involving more than 2,300 medical institutions and 10,000 employees worldwide. In July 2017, as a subsidiary of BGI Group, BGI Genomics (300676.SZ) officially began trading on the Shenzhen Stock Exchange.